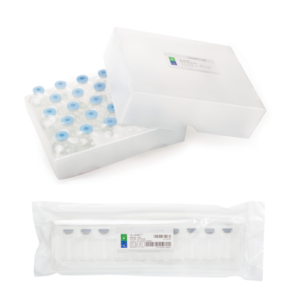
Pre-assembled Sterile Vials
Our pre-assembled sterile vials are supplied ready-to-use with vial(RTU vial), stopper, and seal pre-fitted, offering a reliable solution for radiopharmaceutical filling and sample dispensing. Flexible small-batch customization—whether you need specific vial sizes, stoppers, or seals, configurations can be tailored to your requirements. This adaptability makes them ideal for laboratories, clinical research, and nuclear medicine centers that demand efficiency and precision.
- Sterile and depyrogenated
- Ready to use
- Neutral Type I glass tubing
- CE Marked(upon request)
- ISO 13485:2016 Certified
- DMFs filed with US FDA
- Manufactured per cGMP standards
Process

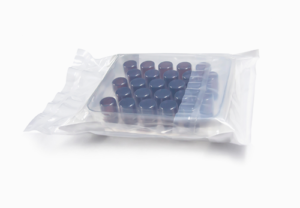
Sterile Open Vials
Our sterile open vials provide a versatile solution for aseptic drug preparation, supporting both manual and automated filling processes. They are widely used for biologics, specialty medicines, radiopharmaceuticals, and sterile compounding in 503A pharmacies as well as large-scale 503B outsourcing facilities, offering unmatched flexibility across research, clinical, and commercial environments.
- Sterile and depyrogenated(upon request)
- Ready to use
- Neutral Type I glass tubing
- ISO 13485:2016 Certified
- Manufactured per cGMP standards
Process

Quality Assurance
Our staff are specially trained with an emphasis on adherence to work instructions and the utmost attention to quality.
All aspects, including material control, manufacturing process, quality control, supplier administration, etc. are defined in a set of Standard Operation Procedures.
A complete GMP quality system has been established.The system has been certified by the US Food and Drug Administration (FDA) (cGMP). It also holds ISO13485 and CE certificate issued by global qualification organizations.


